Why - and when - are they round?
Splashing water and soap bubbles, drops of rain or dew, condensation on the cold surface of a glass, on which to write with the tip of a finger. Games that we began to love as children and that since then, have not yet lost their charm. Games that we are 'borrowing' from the so-called 'capillary forces'. The exploration of the world of capillary forces is an inexhaustible source of wonderful surprises and amazement (not only mathematical).
If their size is small enough, the drops of water or soap bubbles provide a very natural example of an almost perfect sphere. And mathematics helps us understand how small the drops should be, in order to achieve this 'ideal' condition.
With a formula that can be frightening, but it is really quite simple:
Capillary length k = square root of (γ / σ)
where γ is the weight per unit volume and σ the 'surface tension'. In the case of water, we find that drops having a diameter of less than 2 mm are almost perfect spheres.
The spherical shape is a manifestation of the empirically observable isoperimetric inequality, a mathematical formula by which we understand that, given a certain volume in space to be enclosed within an area, the sphere is the form that will provide the minimum area (compared to, for example, a cube or a pyramid).
It is said in these cases that the sphere minimizes the area. And, wonder of nature, the spherical surface is the one that requires the smallest capillary energy- which is proportional to it on the basis of a proportionality constant given by the surface tension σ - that is, in a way, the 'effort' made by the drop in order to keep itself together and enclose a given volume.
When the size of the drop begins to exceed that value of k (which in the case of water is 2 mm), the forces of gravity b egin to be felt, and consequently the equilibrium spherical shape of the drop is warped.
Quite a dynamic drop
Alighting on a solid surface, a drop of 1 mm in diameter 'clings' to it, it loses its round silhouette and takes the form of a spherical cap. And clinging, in many cases, can be really strong. The drops of rain, for example, manage to stay balanced on a glass surface, even if it is vertical. As in the case of a window glass.
However, by treating the surface appropriately, perhaps by making it more rough (with some micrometer high asperities) and hydrophobic (for example, covering it with teflon), the drop will 'yield', rise and remain suspended almost like a fakir lying on a bed of nails.
In these conditions, the drop will again be a near-perfect sphere. Moreover, just like a ball of glass it will begin to roll as soon as the surface is tilted by even a few degrees. The reason is intuitively simple: if the area of contact between drop and solid is reduced to only the tips of the asperities, then the adhesion forces that keep the drop attached become very small.
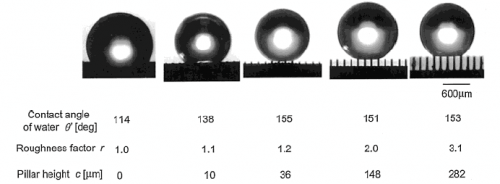
Figure 1: Water droplets on solid surfaces covered with a film of Teflon
There are many surface treatments that make the drops roll off instead of remaining attached. Some are just potential, while others are already on the market. Here are some examples: stain-proof fabric, anti-graffiti coatings, glasses that do not fog up, windshields that do not need wipers.
The quantitative modelling of wetting properties of rough surfaces can benefit greatly from the use of advanced mathematical techniques.
To better understand what happens in reality, the starting point is that the drop is in equilibrium when its total energy reaches its minimum possible value. Through tools and calculation techniques of variations, some mathematicians have first found the formula to calculate the size of the contact angle between the fluid drop and the surface, at which it reaches the minimum energy. This angle, called the Young angle, depends on the degree of 'undulation" of the surface as well as, of course, the types of solid and fluid that get in contact. And this angle determines the degree of water repellency of the surface. A drop may however remain in balance, even if it's not in its minimum energy configuration. This situation may take place with a more or less ample range of contact angles with the surface. The range of possible contact angles between the liquid and the surface - in which it is still possible to maintain the balance - is called hysteresis.
Mathematicians managed to explain how the amplitude of the hysteresis depends on asperities and what is the 'threshold of asperity' of the surface beyond which the drop begins to move and roll.
The conclusion was that a high level of asperity is conducive to little adhesion and rolling of the drops: by increasing roughness until it reaches a critical value, the drops tend to gradually climb over the asperities and to assume a nearly spherical shape due to capillary tension, while the hysteresis decreases simultaneously. Beyond this critical threshold, the drops start to roll. In this case the amplitude of the hysteresis is small, i.e. there are small adhesion forces and therefore the weight of the drop makes it move. From a mathematical standpoint, this is seen through the technique of homogenization. It begins with a problem with a few macroscopic tips, then the number of tips is increased, while their base is narrowed. Without having to compute the individual problems, it is possible to obtain a quantitative prediction of the shape of the droplets in the presence of millions of tiny tips.
The mathematics of capillary phenomena is just one possible example of "everyday math". Natural phenomena accessible with "naive" observation techniques is still a fruitful source of inspiration for research, science and technology. Provided one approaches them with the curiosity and open-mindedness of a child.
P.G. de Gennes: Soft Matter. 1991 Nobel prize lecture: http://nobelprize.org/nobel_prizes/physics/laureates/1991/gennes-lecture.... de Gennes, F. Brochard-Wyart, D. Quere: Gouttes, bulles, perles et ondes, Belin, Paris 2005.
G. Alberti, A. DeSimone: Wetting of rough surfaces: a homogenization approach. Proc. Royal Society A , Vol. 461, p.79 (2005).